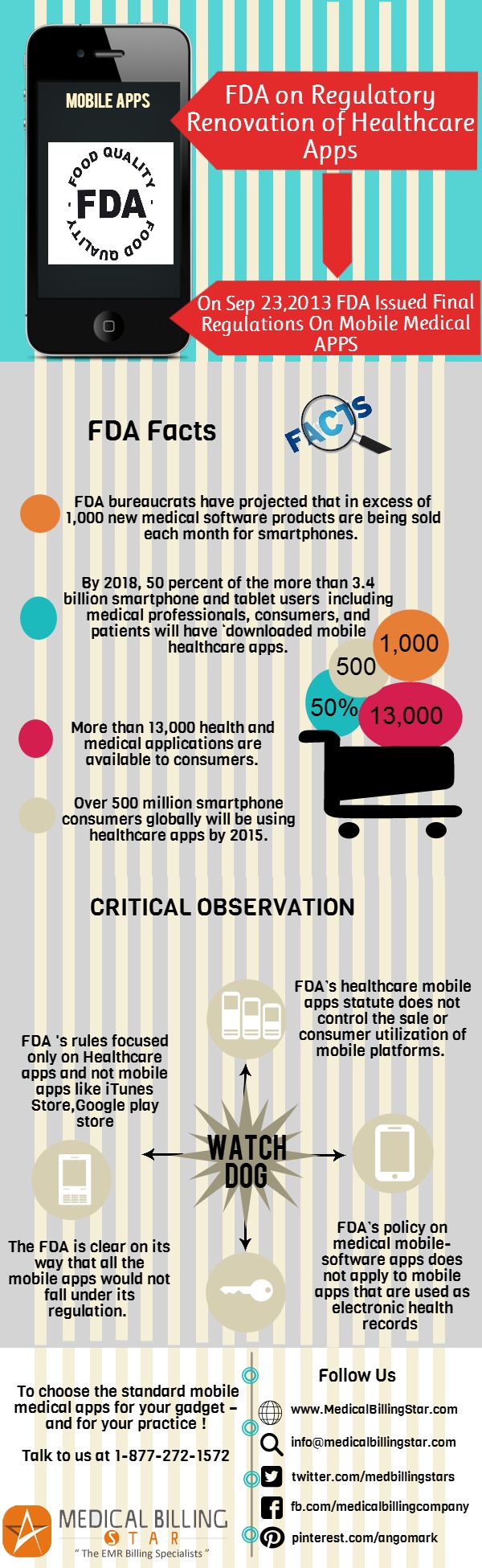
Smartphones and other mobile gadgets have become part and parcel of daily life in Unites States. The ubiquitous espousal and use of mobile apps is unbolting ground-breaking techniques to liven up the health care domain. Very recently, on Sept. 23, 2013, the U.S. FDA (United States – Food and Drug Administration), the federal body for regulating food, drugs and biomedical devices – has issued a final regulation on mobile medical applications (apps), to take a whack at the healthcare mobile technology while preserving the consumer safety.
On the eyes of Watch Dog
“Some mobile apps carry minimal risks to consumer or patients, but others can carry significant risks if they do not operate correctly. The FDA’s tailored policy protects patients while encouraging innovation,”
– Jeffrey Shuren, M.D., J.D., Director of the FDA’s Center for Devices and Radiological Health.
The FDA is clear on its way that all the mobile apps would not fall under its regulation. Mainly mobile apps that help people bolster their health and support the patients in monitoring and improving their health may fall under the FDA’s control.
FDA’s regulations eye on the mobile apps that propose the use in the diagnosis of disease or other health ailments, or in the cure, alleviation, treatment, or prevention of disease or disorders. Besides, apps identifying pills, performing medical calculations, etc. may also come under FDA’s scrutiny. Some of the apps that may meet this rule are listed in the FDA’s official page. The guidance document to know the regulatory pathway of FDA for Class III (high-risk) to Class I (low-risk) mobile medical apps is available right at the FDA website.
The Out-of-Scrutiny Catalog
Mobile devices : FDA’s healthcare mobile apps statute does not control the sale or consumer utilization of mobile platforms.
Apps Stores : FDA’s healthcare mobile apps rule does not mull over companies that solely deal out mobile apps (e.g.: iTunes App store, Google Play store, etc), to be medical device manufacturers.
EHRs : FDA’s policy on medical mobile-software apps does not apply to mobile apps that are used as electronic health records (EHRs) or personal health record system.
The Fact Coffer
- As the apps innovation is propagating at an awe-inspiring pace, there are now more than 13,000 health and medical applications available to consumers and a further 5,000 marketed to healthcare professionals.
- FDA bureaucrats have projected that in excess of 1,000 new medical software products are being sold each month for smartphones.
- The mobile software industry guesstimates that about 500 million smartphone consumers globally will be using healthcare apps by 2015.
- By 2018, 50 percent of the more than 3.4 billion smartphone and tablet users, including medical professionals, consumers, and patients will have ‘downloaded mobile healthcare apps’.
Talk to us to choose the standard mobile medical apps for your gadget – and for your practice !
Leave a Reply